Industrial Sonomechanics, LLC (ISM), offers bench and industrial-scale high-power ultrasonic processors for the production of nanoemulsions. The processors are based on our patented Barbell Horn® Ultrasonic Technology (BHUT).
Overview
The production of nanoemulsions requires significant energy depositions and strong shear forces that can overcome the interfacial tension during the finely dispersed droplet formation. Although methods that do not involve high shear forces exist, they are not applicable to industrial production because they require elevated surfactant concentrations and involve complex preparation procedures. Industrial Sonomechanics® (ISM) offers high-amplitude ultrasonic processors for the production of nanoemulsion. The processors are based on our patented Barbell Horn® Ultrasonic Technology (BHUT), which makes it possible to tremendously intensify the manufacturing processes and guarantees reproducible and predictable results at any scale of operation.
Case Studies & Application Reports
Nanoemulsions Used for Parenteral Nutrition
Drug Carrier Liposomes and Nanoemulsions
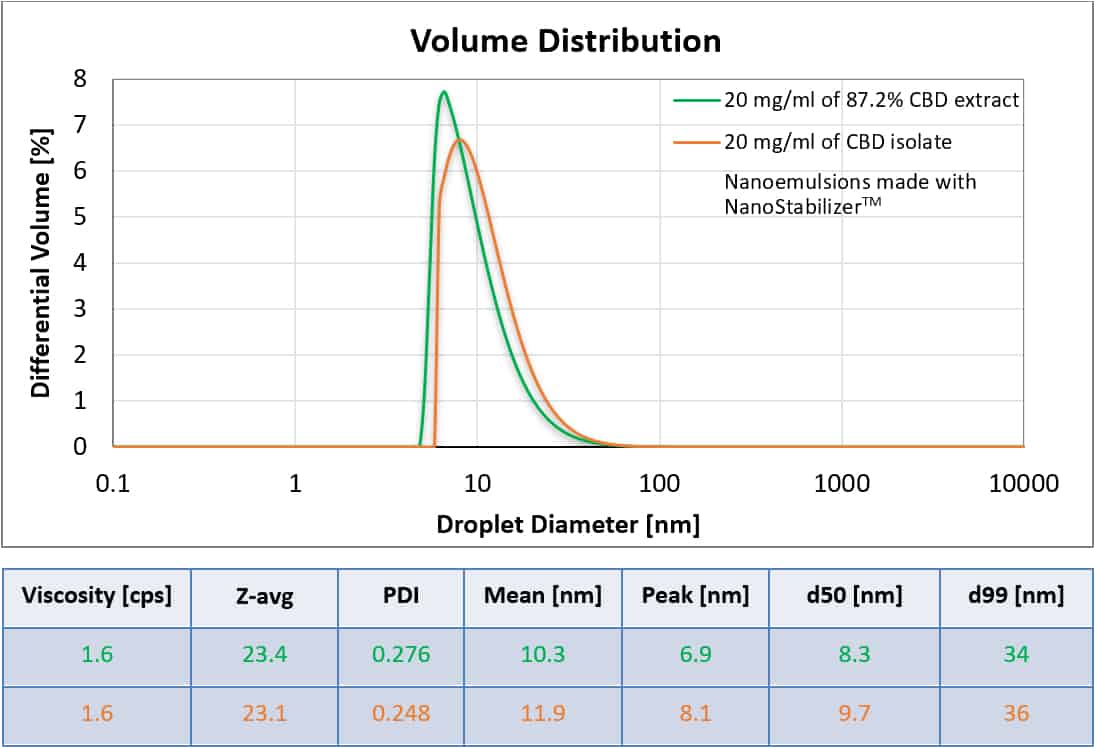
All-Natural CBD Extract Nanoemulsion
Scaling Up the Production of a Nanoemulsion with BHUT
Blog Posts
12/19/23
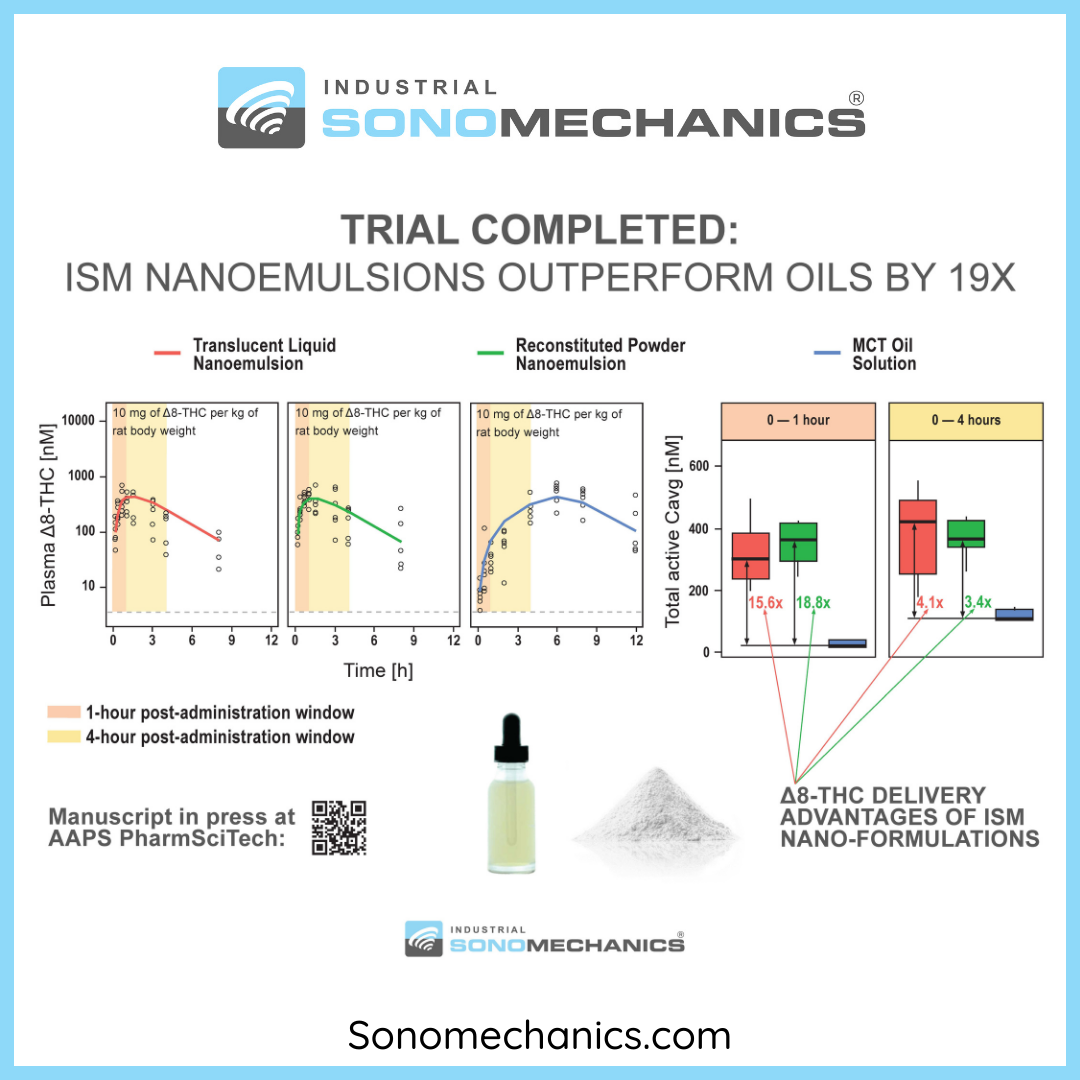
ISM Publishes a Groundbreaking PK Study of Two Δ8-THC Nanoemulsions
Industrial Sonomechanics® (ISM) has recently completed a groundbreak... Read more...
Customer Testimonials
Khara Cartagena, Cannabis and Hemp Productions May 2016
“We recently purchased a BSP-1200 system from ISM and were very impressed with its performance. The word got out, and one of our customers came to our facility to perform CBD-oil emulsion tests with the system. Though he was initially skeptical, after running the machine for only 1 min he got far better results to what he was able to achieve in over an hour with his conventional ultrasonic processor. He absolutely loved the BSP-1200 and bought it off from us that same day to take to his facility in CA! We have now decided to go straight for ISM’s production-scale ISP-3000 processor.”