Industrial Sonomechanics (ISM), offers ultrasonic processors for the production of nanosized drug crystals. Our patented Barbell Horn® Ultrasonic Technology (BHUT) allows us to scale up processes from the lab to the industrial scale, guaranteeing reproducible and predictable results.
Overview
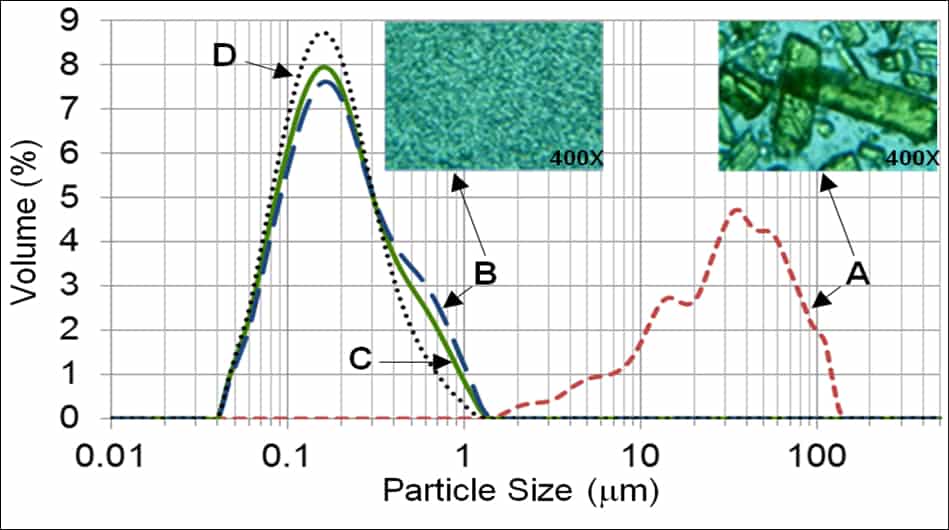
Many biologically active compounds (e.g., drugs and nutraceuticals) exhibit poor water solubility, which complicates their delivery to the blood stream and reduces the associated bioavailability. Top-down nano-crystallization (particle size reduction to the nanometer range) of these substances increases their aqueous dissolution rate and solubility, which results in improved bioavailability, accelerated onset of action, and decreased potential of harmful side-effects.
Case Studies & Application Reports
Ultrasonic Nano-crystallization of Nifedipine
Nano-crystallization for Improved Drug Delivery
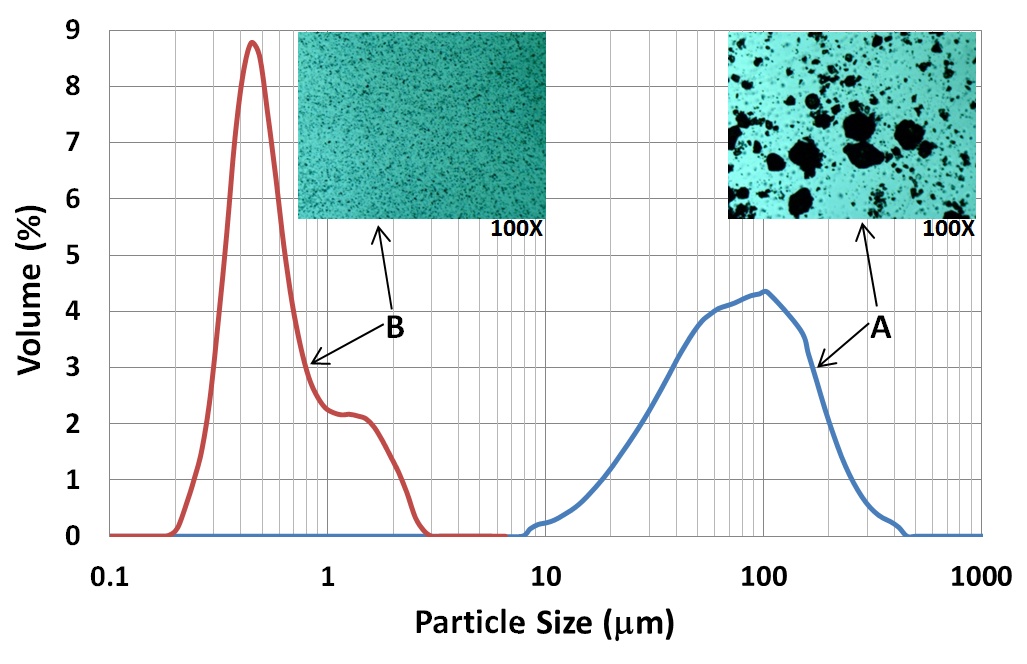
Sonofragmentation of Excipient Crystals
Blog Posts
12/14/17
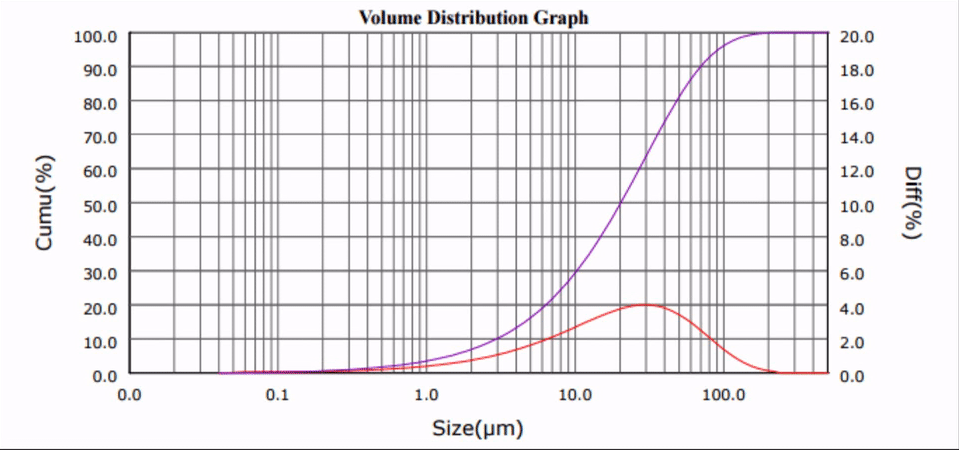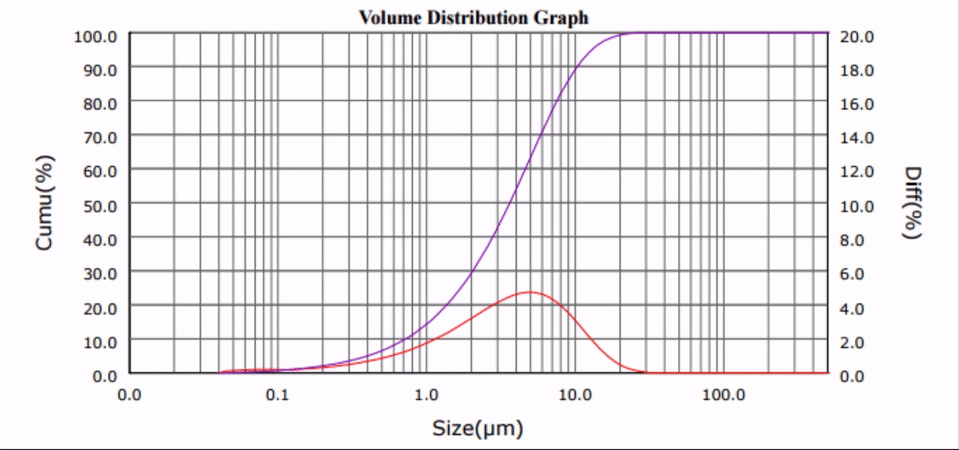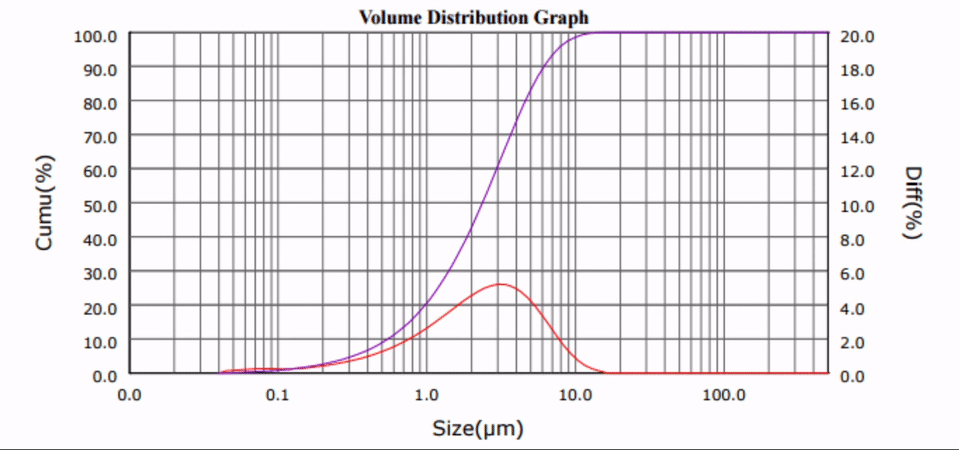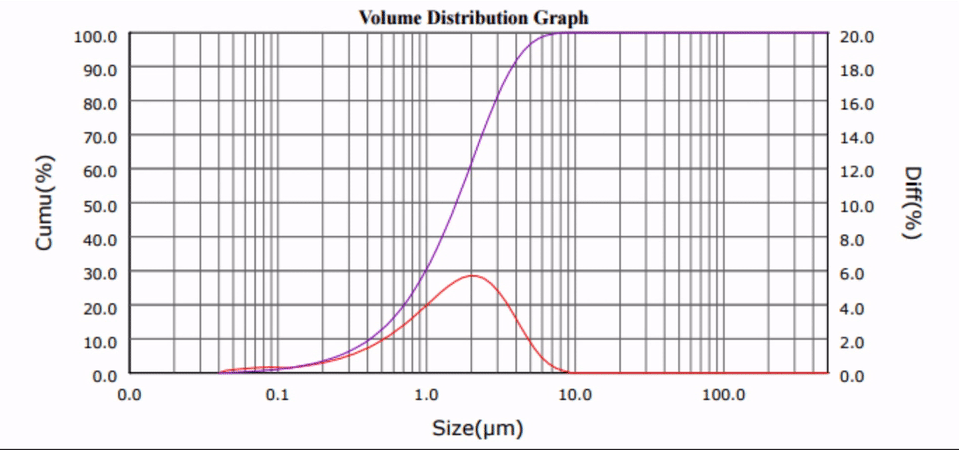
Ultrasonic Nano-Crystallization of Salsalate for Improved Bioavailability
Salsalate is part of a class of naturally occurring chemicals known ...
Read more...
10/06/16
Ultrasonic Dispersing and Wet-milling of Calcium Carbonate (CaCO3)
Cement, and its paved derivative, concrete, is a widely-consumed mat... Read more...
01/02/16
Acoustic Cavitation: The Driving Force Behind Ultrasonic Processing
Liquids exposed to high-intensity ultrasound can undergo acoustic ca... Read more...
Customer Testimonials
Khara Cartagena, Cannabis and Hemp Productions May 2016
“We recently purchased a BSP-1200 system from ISM and were very impressed with its performance. The word got out, and one of our customers came to our facility to perform CBD-oil emulsion tests with the system. Though he was initially skeptical, after running the machine for only 1 min he got far better results to what he was able to achieve in over an hour with his conventional ultrasonic processor. He absolutely loved the BSP-1200 and bought it off from us that same day to take to his facility in CA! We have now decided to go straight for ISM’s production-scale ISP-3000 processor.”